Abstract
INTRODUCTION: Allogeneic hematopoietic cell transplantation (HCT) is a diffuse curative option for transfusion dependent thalassemia (TDT) and sickle cell disease (SCD). To verify transplant activity, distribution, demography, policies and outcomes the Hemoglobinopathy Registry was established inside the European Group for Blood and Marrow Transplantation (EBMT). After a previous analysis limited to TDT for the 2000-2010 period data (BMT 2016; 51:536-41), we performed an updated report considering TDT and SCD patients transplanted in the last eighteen years (years 2000-2017).
METHODS: Data on pediatric patients transplanted between Jan 1st, 2000 through Dec 31st, 2017 were extracted by the EBMT promise Hemoglobinopathy registry database. Only first transplants were considered. Data are expressed as median with range unless specifically indicated. Survival probabilities were calculated with the method of Kaplan and Meier and expressed as means and 95% confidence intervals (95%CI). Differences between survival probabilities were tested by means of the log-rank test.
RESULTS: In the above-specified period 3856 consecutive pediatric patients affected by TDT (2936, 76%) or SCD (920, 24%) were transplanted in 166 HCT centers distributed in 36 countries in Europe, Asia and Africa. Median age at transplant was 7.2 years (range 0.48-17.9). 3342 (87%) transplants were performed on patients <14 years, 514 (13%) on patients aged 14-17.9 years. 2593 (67%) patients received transplant from an HLA identical sibling donor.
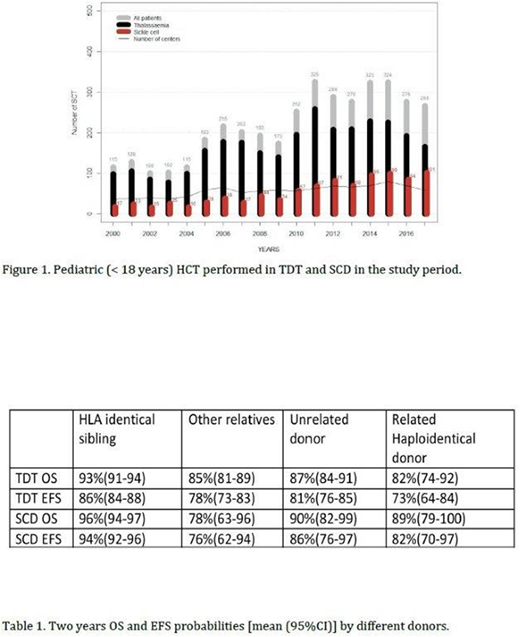
Figure 1 reports pediatric transplant activity inside the EBMT showing an increased numbers of patients transplanted after year 2010.
After a median follow up of 24 months, the 2 years overall survival (OS) and event-free survival (EFS) were 91% (95%CI 90-92) and 86% (95%CI 85-87) for the entire population.
In TDT, OS and EFS were 90% (95%CI 89-92) and 84% (95%CI 82-85), respectively.
In SCD, OS and EFS were 94% (95%CI 92-96) and 92% (95%CI 90-94), respectively.
In both diseases no outcome difference was recorded on the basis of year of transplant (data not shown).
Source of hematopoietic cells was bone marrow in 70% of TDT transplants and 81% of SCD transplants. In both diseases better results were recorded with the use of bone marrow versus peripheral blood [OS 91% versus 85% (P< 0.001); 95% versus 84% (P= 0.004) for TDT and SCD, respectively]. Similar results were recorded when EFS was analyzed.
176 patients received hematopoietic cells from single cord blood donors. Of them only 21 were from an unrelated donor. 119 patients received cord blood + bone marrow. Results of related HLA identical cord blood were similar to that of HLA identical related bone marrow (data not shown).
Transplantation from an HLA identical sibling offered the best results in OS and EFS compared to other donor options (P< 0.001), both for TDT and SCD. Transplant results by donor type are reported in table 1.
The threshold age for optimal transplant outcomes is confirmed 14 years, with OS 92% (95%CI 91-93), EFS 87% (95%CI 85-88) versus OS 85% (95%CI 82-89), EFS 81% (95%CI 77-85) ( P<0.001) comparing younger versus older patients in the entire populations. These differences were confirmed either in TDT and SCD.
The two years incidence of cumulative extensive chronic graft versus host disease was 4.1% in TDT and 4.4 % in SCD (P=ns).
CONCLUSIONS: Allogeneic HCT for TDT and SCD is a widely available curative approach; the procedure has been increasing and internationally performed during the years with excellent results. The emerging gene therapy approach will have to be compared to these well-established results.
Zecca:Chimerix: Honoraria. Forni:Novartis: Other: travel expenses, Research Funding; Celgene: Research Funding; Roche: Consultancy; Shire: Research Funding; Apopharma: Other: DSM Board. Bader:Medac: Patents & Royalties, Research Funding; Neovii: Research Funding; Novartis: Consultancy, Speakers Bureau; Riemser: Research Funding; Cellgene: Consultancy. Angelucci:Vertex Pharmaceuticals Incorporated (MA) and CRISPR CAS9 Therapeutics AG (CH): Other: Chair DMC; Novartis: Honoraria, Other: Chair Steering Comiittee TELESTO Protocol; Roche Italy: Other: Local (national) advisory board; Jazz Pharmaceuticals Italy: Other: Local ( national) advisory board; Celgene: Honoraria, Other: Chair DMC.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal